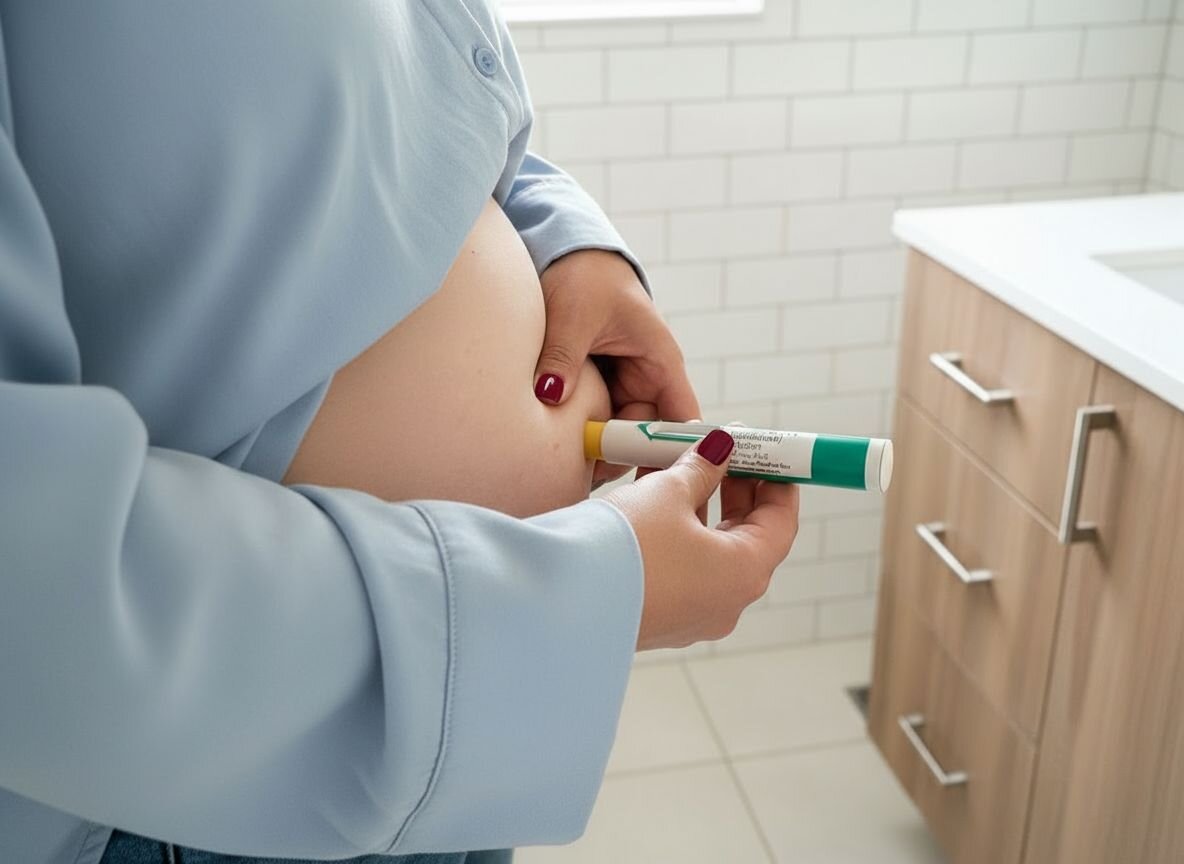
Many patients prescribed Dupixent® (dupilumab) for eczema, asthma, or related conditions have developed rare forms of skin cancer, which are now under FDA safety review.
If you or a loved one used Dupixent for 30 days or more, you may be entitled to significant compensation.